
Membrane Processes
Membrane processes are defined in physical ways for solvent separation from soluble salts using semi-permeable membranes. These processes have made a lot of progress in recent years. The history of using the membrane for straightening back to the early twentieth century. In the third decade of the twentieth century membranes were used to isolate, purify or condense solutions, especially fluids containing microorganisms. The evolutionary process of this phenomenon continued with the ongoing research on the manufacture of membranes and the recognition of the process over time, which is currently considered as one of the main methods of sweetening seawater.
Types of membrane processes
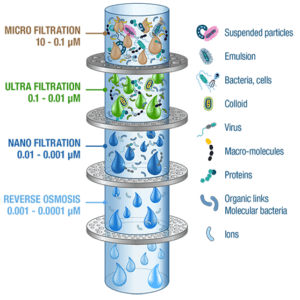
Membrane processes are called by the following names, based on the size of the smallest particle affected by the pressure of the membrane:
A) Micro Filtration
B) Ultra Filtration
C) Nano Filtration
Reverse Osmosis (Hyper Filtration)

In a membrane process, two phases are typically separated by a third phase, the membrane. The membrane determines the phenomenon of mass transfer. Each of the two phases is a solution of different components, some of these soluble components are transported through the membrane over other components. If the mass transfer proceeds, one of the phases is covered by hidden passages and the remainder of these fragments. The two main properties of the transfer, which are applied by the membrane, are:
A) Permeability or permeability
B) selectivity
All materials that act as membranes, have the characteristics of passing different materials selectively.
Membrane processes can benefit from various propulsion forces for separation. Mass transfer in a membrane can be accomplished by penetration or displacement phenomena. The phenomenon of mass transfer is due to the difference in electrical potential, concentration, pressure or temperature.
Membrane processes have the following benefits:
A. Energy savings due to the lack of phase change.
B) Decrease space required due to the low volume of membrane modules.
C) faster process due to the membrane’s thinness and high mass transfer velocity.
D) The ability to perform at low temperatures is an advantage in heat-sensitive solutions.
E) Low investment cost compared to other global methods.
(C) Ease of system expansion.
Q) Ability to build in different shapes and sizes.
Membranes have a long life. The useful life of ultrafiltration membranes for more than two years and the useful life of reverse osmosis membranes have been reported for more than five years. Membranes are made of various organic or mineral materials. These materials include polymers, ceramics, metals and fluids, which are widely used polymer materials. The function of the membrane is the origin of the membrane’s membrane selection in the design of a membrane process. The membrane function is characterized by its physical and chemical properties.
Chemical Properties and Physical Properties of the Membrane
The chemical properties of the membrane include its reactivity with other materials, surface charge and the ability to absorb other objects. The physical properties of the membrane are the size, shape and type of holes in the membrane, mechanical strength and thermal resistance. Water-solubility or water membrane is due to its physical and chemical properties. The most important variables that determine the physical and chemical properties of a membrane include the raw material used, the molding process and the molding process.
.
Polymer membranes are used more than other membranes. The application of each membrane depends on the environmental conditions. For example membranes made from cellulose nitrate or cellulose acetate are sensitive to heat, chemicals and biological materials. In order to prevent their deterioration, the pH of the acid environment should be between 4 and 6.5 and the temperature of the membrane should be at a standard temperature. The membranes produced from cellulose derivatives in alkaline environments are rapidly hydrolyzed. Teflon membranes (PTFEs) have more resistance than cellulosic membranes. In acid and game environments and chemicals, except hydrocarbons, they are resistant to high temperatures. These membranes have a temperature range of 100 to 300 ° C. Teflon membranes are reinforced mechanically by polyethylene holders so that they can not be damaged if they are displaced. Membranes made of polyvinyl chloride (PVC), in pure form or in combination with other polymers, have a good resistance to acids and strong bases, but they are softened at temperatures in excess of 65 ° C.
After use, the membrane needs to be cleaned as soon as possible. This is done with chemicals. The choice of a cleaner chemical depends on the polymer used in the membrane. For example, membranes made of polyamide, which have a good resistance to solvents and have a sterile autoclave, are highly sensitive to chlorine in chemicals, and therefore chloride materials can not be used to clean these membranes. Made
The membrane structure is called membrane morphology and plays an important role in its function.
Solid membranes are in two main structures:
A) Porous membranes
B) Non-porous membranes
In porous membranes, the size of the holes and their distribution are different. The membranes are classified according to the size of their cavities.
A. Large holes: membranes with a mean pore size of more than 20 nm.
B) Medium cavities: membranes with a moderate cavity size



