Copper and its importance

Origin and Importance of Copper
Copper (Cu) is the first element in group IB of the periodic table. It has an atomic number of 29, an atomic weight of 63.54, and valences of 1 and 2. The average abundance of Cu in the Earth's crust is 68 ppm, in soil it is 9 to 33 ppm, in running water it is 4 to 12 μg/L, and in groundwater it is <0.1 mg/L. Copper exists in its elemental state, but is also found in many minerals, the most important of which are sulfide compounds (such as chalcopyrite), as well as oxides and carbonates.
Copper is widely used in electrical wiring, roofing materials, various alloys, pigments, cookware, plumbing, and the chemical industry. Copper salts are used in water supply systems to control biological growth in tanks and distribution pipes and to catalyze the oxidation of manganese. Copper forms a number of complexes with organic and inorganic ligands in natural waters.
Common types of water include Cu2+, Cu(OH)2, and CuHCO3+. Corrosion of copper-containing alloys in pipe fittings may introduce measurable amounts of copper into the water in a piping system.
Copper is an essential element for plants and animals. Some of its compounds are toxic if ingested or inhaled. The Food and Agriculture Organization of the United Nations recommends a maximum level of copper in waters used for irrigation of agricultural crops of 200 μg/L.
Method Selection
Atomic absorption spectrometric methods (Sections 3111B and C), inductively coupled plasma methods (Sections 3120 and 3125), and the Neocuproine method (CuB-3500) are recommended because of their lack of interference from interfering substances. The electrothermal atomic absorption method (Section 3113B) has also been used successfully with a suitable matrix modifier. The Bathocuproine (3500-Cu.C) method can be used for drinking water.
Sampling and Storage
Copper ions tend to adsorb to the surfaces of sample containers. Therefore, analyze samples as soon as possible after collection. If storage is necessary, use 0.5 mL 1 + 1 HCl/100 mL sample to prevent this adsorption, or acidify the medium to pH <2 with HNO3.
Neocuproine Method
General Discussion
Principles: Copper ions (Cu+) react in neutral or slightly acidic solution with 2,9-dimethyl-1,10-phenanthroline (neocuproine) to form a complex in which 2 moles of neocuproine bind to 1 mole of Cu+ ions. This complex can be extracted with a number of organic solvents, including a chloroform-methanol mixture (CHCl3-CH3OH) to give a yellow solution with a molar absorbance of about 8000 at 457 nm.
The reaction is almost specific for copper; the color is present up to a concentration of 0.2 mg Cu/25 ml solvent, according to Beer's law; the full color range is achieved when the pH of the aqueous solution is between 3 and 9. The color is stable for several days in CHCl3-CH3OH. The sample is treated with hydroxylamine hydrochloride to convert the cupric ions to cupric ions.
Sodium citrate is used for complex metal ions that may precipitate when the pH is increased. The pH is adjusted to 4 to 6 with NH4OH, a solution of neocorvus in methanol is added, and the resulting complexes are extracted as CHCl3. After diluting CHCl3 to an accurate volume with CH3OH, the absorbance of the solution is measured at 4571 nm.
Interference: Large amounts of chromium and tin may interfere. Avoid interference from chromium by adding sulfuric acid to reduce chromate and complexed chromium ions. In the presence of large amounts of tin or excessive amounts of other oxidizing ions, use excess hydroxylamine hydrochloride solution. Cyanide, sulfide, and organic materials interfere, but can be removed by digestion.
Minimum detectable concentration: The minimum detectable concentration corresponds to an absorbance of 0.01 or a transmission of 98%, using 3 μg of copper when using a 1 cm cell and 0.6 μg of copper when using a 5 cm cell.
Supplies
Colorimetric Equipment:
One of the following is required:
- Spectrophotometer for use at 4571 nm, providing a light path of 1 cm or more.
- Filter photometer, providing a light path of 1 cm or more and equipped with narrow violet filters with maximum transmission in the range of 450 to 460 nm.
Separating funnels, 125 ml, Squibb shape, with glass or TFE tubing and probe.
Reagents
- Double-distilled water, copper-free: Since most ordinary distilled water contains detectable amounts of copper, use double-distilled water for the preparation of all reagents and solutions, which is provided by redistilling distilled water in a resistant glass, or distilled water transferred from an ion exchange unit.
- Copper stock solution: To 200.0 mg of copper wire or foil in a 250-mL conical flask, add 10 mL of water and 5 mL of concentrated HNO3. After the reaction has slowed, heat gently to complete the dissolution of the copper and boil to remove nitrous oxide, taking precautions to avoid loss of copper. Cool the solution, transfer approximately 50 mL of water quantitatively to a 1-L volumetric flask, and dilute with water; 1 mL = 200 μg cc.
- Copper standard solution: Dilute 50.00 mL of the copper stock solution to 500 mL with water; 1.00 mL = 20.0 μg cc.
- Sulfuric acid, H2SO4, concentrated.
- Hydroxylamine hydrochloride solution: Dissolve 50 g NH2OH HCl in 450 ml water.
- Sodium citrate solution: Dissolve 150 g Na3C6H5O7 2H2O in 400 ml water. Add 5 ml NH2OH HCl solution and 10 ml neocuproine reagent. Extract the extract with 50 ml calcium chloride to remove copper impurities and remove the CHCl3 layer.
- Ammonium hydroxide, NH4OH, 5N: Dilute 330 ml of concentrated NH4OH (28-29%) to a volume of 1000 ml with water. Store in a polyethylene bottle.
- Congo red paper or other pH test paper indicates a color change in the pH range of 4 to 6. I. Neocuproine Reagent: Dissolve 100 mg of 2,9-dimethyl-1,10 phenanthroline hemihydrate*#(106) in 100 mL of methanol. This solution is stable under normal storage conditions for one month or more.
- Chloroform, CHCl3: Avoid using materials stored in containers with metal caps or the material will be redistilled.
- Methanol, CH3OH, reagent grade.
- Nitric acid, HNO3, concentrated.
- Hydrochloric acid, HCl, concentrated.
Procedure
Prepare calibration curve: Pipette 50 mL of water into a 125 mL separatory funnel to use as a blank. Prepare standards by transferring 1.00 to 10.00 mL (20 to 200 μg/ha) of copper standard solutions by pipette into a series of 125 mL separatory funnels and make up to 50 mL with water. Add 1 mL of concentrated H2SO4 and use the extraction procedure (below 4(B).
Plot a calibration curve by plotting absorbance versus micrograms of copper.
To prepare a calibration curve for lower amounts of copper, dilute 0.10 mL of the copper standard solution to 100 mL. Prepare 1.00 to 10.00 mL of this diluted standard in the same manner as previously described, but use a 5-cm cell for absorbance measurements.
Sample preparation: Transfer 100 mL of the sample to a 250 mL beaker, add 1 mL of concentrated H2SO4 and 5 mL of concentrated HNO3. Add a little anti-boiling agent and evaporate carefully until white SO3 condenses on the walls of a hot plate. If a colored solution remains, cool it, add 5 mL of concentrated HNO3, and re-evaporate. Evaporate until a thick layer of white foam forms. Repeat this process, if necessary, until the solution is colorless.
Cool the solution, add about 80 ml of water and bring to the boil. Cool again and pour into a 100 ml volumetric flask. Make up to 100 ml with the same water used to rinse the beaker and filter.
Transfer 50.0 ml of the solution containing 4 to 200 µg of copper from the solution obtained in the initial preparation into a 125 ml separating funnel. If necessary, make up to 50 ml with water. Add 5 ml of NH2OH·HCl solution and 10 ml of sodium citrate solution and mix thoroughly. Adjust the pH to about 4 by adding 1 ml of NH4OH until the Congo red paper turns completely red or (other pH test papers indicate a value between 4 and 6).
Add 10 ml of neocuproine and 10 ml of CHCl3. Cap the flask and shake gently for 30 seconds or more to extract the copper-neocuproin complex in CHCl3. Allow the mixture to separate into two separate phases and transfer the lower CHCl3 layer to a 25 mL voltammetric flask, making sure not to transfer any volume of the aqueous phase. Repeat the aqueous phase extraction by adding an additional 10 mL of CHCl3 and combine the extracted phases. Dilute the extracted phases to a volume of 25 mL with CH3OH, cap and mix thoroughly.
Transfer an appropriate portion of the extracted phase to a suitable absorption cell (1 cm for 40 to 200 μg and 5 cm for lower amounts) and measure the absorbance at 4571 nm or with a 450 to 460 nm filter. Use a blank prepared by adding 50 mL of water after the digestion and analysis steps are complete.
Determine the micrograms of copper in the final solution, given the appropriate calibration curve.
Calculations

Resources
SMITH, G.F. & W.H. MCCURDY. 1952. 2,9-Dimethyl-1,10-phenanthroline: New specific in spectrophotometric determination of copper. Anal. Chem. 24:371.
LUKE, C.L. & M.E. CAMPBELL. 1953. Determination of impurities in germanium and silicon. Anal. Chem. 25:1586.
GAHLER, A.R. 1954. Colorimetric determination of copper with neocuproine. Anal. Chem. 26:577.
FULTON, J.W. & J. HASTINGS. 1956. Photometric determinations of copper in aluminum and lead-tin solder with neocuproine. Anal. Chem. 28:174.
FRANK, A.J., A.B. GOULSTON & A.A. DEACUTIS. 1957. Spectrophotometric determination of copper in titanium. Anal. Chem. 29:750.
3500-Cu C. Bathocuproineروش
General Discussion
Principles: Copper ion forms a water-soluble orange chelate with bathocuproine disulfonate (2,9-dimethyl-4,7-diphenyl-1,10-phenanthrene sulfonic acid, dodecyl salt).
Although the color is observed over a pH range of 3.5 to 11.0, the recommended pH is between 4 and 5. The sample is buffered to pH 4.3 and reduced with hydroxylamine hydrochloride. The absorbance is measured at 4841 nm. The method can be used for copper concentrations down to at least 5 mg/L with a sensitivity of 20 μg/L.
Interference: The following substances do not interfere at the maximum levels stated, with an error of less than ± 2%:
Substance | Concentration mg/L |
Cations | |
Aluminum | 100 |
Beryllium | 10 |
Cadmium | 100 |
Calcium | 1000 |
Chromium (III) | 10 |
Cobalt (II) | 5 |
Iron (II) | 100 |
Iron (III) | 100 |
Lithium | 500 |
Magnesium | 100 |
Manganese (II) | 500 |
Nickel (II) | 500 |
Sodium | 1000 |
Strontium | 200 |
Thorium (IV) | 100 |
Zinc | 200 |
Anions | |
Chlorate | 1000 |
Chloride | 1000 |
Fluoride | 500 |
Nitrate | 200 |
Nitrite | 200 |
Orthophosphate | 1000 |
Perchlorate | 1000 |
Sulfate | 1000 |
Compounds | |
Residual chlorine | 1 |
Linear alkylate sulfonate (LAS) | 40 |
Cyanide, tricyanide, persulfate, and EDTA may also interfere.
Minimum detectable concentration: 20 μg/L with 5 cm cell.
Equipment
Colorimetric equipment: One of the following with a 1 to 5 cm light path (unless Nessler tubes are used):
- Spectrophotometer for use at 4841 nm.
- Filter photometer, equipped with a blue-green filter showing a maximum near 4841 nm.
- Nessler tubes, identical, 100 mL, long form.
Acid-washed containers: Wash all containers with concentrated HCl solution and then with copper-free water.
Reagents
- Copper-free water: See Method B, 3a.
- Copper stock solution: As directed in Method B, 3b, but use 20.00 mg of copper wire or foil; 1.00 mL = 20.00 μg of copper.
- Copper standard solution: Dilute 250 mL of copper stock solution to 1000 mL with water. 1.00 mL = 5.00 μg of copper. Prepare daily.
- Hydrochloric acid, HCl, 1 + 1.
- Hydroxylamine hydrochloride solution: See Method B, 3e.
- Sodium citrate solution: Dissolve 300 g of Na3C6H5O7 2H2O in water and make up to 1000 mL.
- Disodium bathocuproine disulfonate solution: Dissolve 1.000 g of C12H4N2(CH3)2(C6H4)2(SO3Na)2 in water and make up to 1000 ml.
Procedure
Pipe 50.0 ml of the sample into a 250 ml Erlenmeyer flask. Prepare a 50.0 ml water blank and a series of 50.0 ml copper standards containing 5.0, 10.0, 15.0, 20.0 and 25.0 µg copper. For the sample, blank and standards, mix 1.00 ml of 1 + 1 HCl solution, 5.00 ml of NH2OH HCl solution, 5.00 ml of sodium citrate solution and 5.00 ml of disodium bathocuproine disulfonate solution after each addition. Transfer to the cell and read the absorbance of the sample against the blank at 4841 nm. Plotting the absorbance points against micrograms of Cu in the standard solutions gives us the calibration curve. Estimate the concentration from the calibration curve.
Calculations
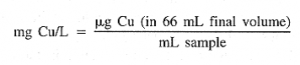 .
.
Precision
A synthetic sample containing 1000 μg Cu/L, 500 μg Al/L, 50 μg Cd/L, 110 μg Cr/L, 300 μg Fe/L, 70 μg Pb/L, 50 μg Mn/L, 150 μg Ag/L, and 650 μg Tin/L was analyzed in 33 laboratories using the Bathocuproine method, with a relative standard deviation of 4.1% and a relative error of 0.3%.
7. Bibliography
SMITH, G.F. & D.H. WILKINS. 1953. New colorimetric reagent specific for copper. Anal. Chem. 25:510.
BORCHARDT, L.G. & J.P. BUTLER. 1957. Determination of trace amounts of copper. Anal. Chem. 29:414.
ZAK, B. 1958. Simple procedure for the single sample determination of serum copper and iron. Clinica Chim. Acta 3:328.
BLAIR, D. & H. DIEHL. 1961. Bathophenanthrolinedisulfonic acid and bathocuproinedisulfonic acid, water soluble reagents for iron and copper. Talanta 7:163.
share :













Submit your opinion
Your email address will not be published.