Corrosion process

Introduction
Corrosion of metals is the process in which metals reach their original oxidation numbers. In fact, corrosion of metals is an oxidation-reduction reaction in which the metal is oxidized by its surroundings.
Typically, oxygen from the air plays the role of the oxidant in these processes. This reaction is also considered a popular and spontaneous reaction in terms of electrochemical reactions.
In other words, this process involves the formation of a galvanic cell in which the metal in question acts as the anode and usually corrodes or loses its function.
The corrosion process causes the deterioration or destruction of the metal. The most common example of corrosion is the formation of rust on steel. Most of the phenomena of the corrosion process are electrochemical in nature and consist of at least two reactions on the surface of the corroding metal.
One of the reactions is oxidation (e.g., dissolution of iron), also called partial anodic reaction. In this regard, tests are performed to measure the amount of iron in the water of the system (systems with circulating water, in boilers, etc.). By obtaining the amount of iron in the water, corrosion and rust, and consequently the formation of deposits in the system, can be prevented or minimized by using anti-corrosion solutions (corrosion inhibitors). Another is a reduction reaction (e.g., reduction of oxygen), also called partial cathodic reaction. The products of electrochemical reactions can react with each other in a non-electrochemical manner and form the final product (e.g., rust). For example, the corrosion of iron to form rust follows the general reaction:
2Fe + 2H2O → 2Fe(OH)2
This reaction involves the dissolution of iron, the reduction of oxygen, and the formation of rust:
Fe → Fe 2+ +2e- (anodic)
2H2O + O2 + 4e- → 4OH– (cathodic)
Fe 2+ + 2OH – → Fe(OH)2(chemical)
Types of Corrosion Process
Uniform Corrosion Process:
Uniform corrosion process is carried out by corrosion attack that is generally evenly distributed over a large area or part of the metal surface. Uniform corrosion causes material loss to failure. This is the most widespread form of corrosion observed.

Pitturing Corrosion Process:
Pitturing corrosion process is a type of localized corrosion by which holes are produced in materials. Pitting corrosion damage is considered more dangerous than uniform corrosion because it is more difficult to predict. Corrosion products often cover the holes, which are often very difficult to detect. A small, narrow hole with minimal metal loss throughout can lead to complete failure of an engineering system.

Crevice Corrosion Process:
Crevice corrosion process is a type of localized corrosion that occurs when a stagnant solution is present in a small (micro) gap. Local chemical changes in gaps (protective areas) such as those under gaskets, insulation materials, surface deposits, detached coatings, threads, flange connections, and fasteners can lead to crevice corrosion.

Galvanic Corrosion Process:
Galvanic corrosion refers to corrosion damage caused when two dissimilar materials are present in a corrosive electrolyte. Galvanic corrosion occurs when two (or more) dissimilar metals are electrically connected to water. When a galvanic couple is formed, one of the metals in the couple becomes the anode and corrosion occurs more rapidly than either alone, while the other becomes the cathode and corrosion occurs more slowly than either alone. Either (or both) metals in the couple may or may not corrode by themselves in seawater.

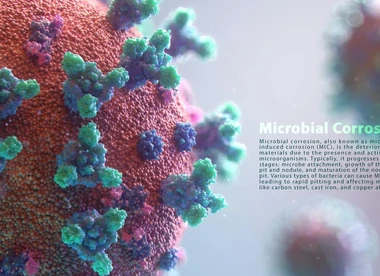
Microbiological Corrosion Process: (MIC)
Microbiological corrosion, or MIC, refers to corrosion caused by biological organisms or microbes. These microbes are classified based on common characteristics such as their byproducts (e.g., sludge production) or the compounds they affect (i.e., sulfur oxidizers). They all fall into one of two groups based on the amount of oxygen they require. One is aerobic (requires oxygen) such as sulfur-oxidizing bacteria and the other is anaerobic (requires little or no oxygen) such as sulfate-reducing bacteria.

Conclusion
We can summarize the importance of corrosion in three words: economy, safety, and protection. If we do not prevent this phenomenon, heavy direct and indirect costs will be imposed on us. In addition to the economic aspects, the sudden destruction of structures due to various types of corrosion can endanger the lives of thousands of people. In general, in electrochemical reactions, two half-reactions take place simultaneously. In one reaction, electrons are produced and in the other, electrons are consumed. For a corrosion reaction to occur, we must have a complete electrochemical circuit. The elements that make up an electrochemical circuit include the cathode (electron consuming component), the anode (electron donating component), and the electron transfer path. In corrosion systems, the electron transfer path is the electrolyte, in other words, the corrosive environment. If any of these elements are not present, the corrosion process will not occur...
share :












Submit your opinion
Your email address will not be published.