The three main enemies of cooling towers: scale, corrosion, biofouling

Scale, Corrosion, and Biofouling: The Three Main Enemies of Cooling Towers
Is your cooling tower losing efficiency? Is your energy consumption increasing or are you worried about sudden failures? Behind these problems lie three main factors: Scale, Corrosion, and Biofouling. These three enemies act secretly and reduce the life of the equipment and increase operating costs.
Controlling these three challenges is the key to optimal performance, long life, and energy savings of cooling systems. In this article, we will comprehensively examine each of these enemies, their causes, identification symptoms, and effective solutions to combat them.
What is a cooling tower and why are these three enemies vital to it?
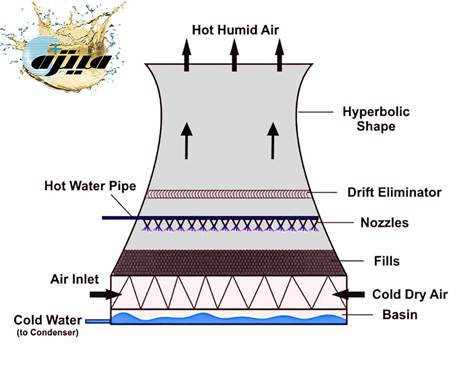
A cooling tower is the beating heart of many industrial processes, central air conditioning systems, and power plants. This system transfers the heat of the process to the environment by circulating water and its contact with air. But this natural process of evaporation increases the concentration of substances dissolved in water and gives rise to three basic problems:
1. Sedimentation: Formation of hard, insulating layers on surfaces.
2. Corrosion: Erosion and destruction of structural metals.
3. Biofouling: Growth of microbial colonies and formation of biofilm.
These three factors not only act alone, but also together and exacerbate the damage.
Scale; Hidden Insulation Wall
What is scale and how does it form?
Scale is a hard, rock-like, thermally insulating layer that forms due to the precipitation of water-soluble salts on hot surfaces or areas with low water flow. The main cause is the presence of hard water with a high concentration of calcium (Ca²⁺) and magnesium (Mg²⁺) ions.
When water evaporates in a cooling tower, the concentration of these salts increases and passes the saturation point. In this condition, insoluble salts such as calcium carbonate (CaCO₃), calcium sulfate (CaSO₄), and silicates begin to form hard crystals on the surface of the tubes and heat exchangers.
What types of deposits are common in cooling towers?
The three main types of deposits in cooling systems are:
- Calcium carbonate (CaCO₃):The most common type of deposit that forms at high pH.
- Calcium sulfate (CaSO₄):Forms under conditions of very high TDS and high temperatures and is harder than calcium carbonate.
- Silica (SiO₂):A very hard and sticky deposit that combines with other salts, making it very difficult to remove.
You can also read the article on examining and controlling sediment in cooling towers.
What are the symptoms and consequences of scale formation?
Reduced thermal efficiency: Scale acts as an insulator and impedes heat transfer. Even a thin layer of 1/8 inch (about 3 mm) can increase energy consumption by up to 40%.
Reduced water flow:Scale in pipes reduces flow diameter and increases pump pressure.
Hot spots:In heat exchangers, poor heat transfer causes the metal to overheat and can lead to cracking.
Under-Deposit Corrosion:Scale acts as a heterogeneous coating, creating an environment with different chemical conditions underneath it that accelerates corrosion.

Deposits inside the cooling tower

Inside the cooling tower after sediment removal

Sample of sediments inside the cooling tower
How to prevent scale formation?
Scale control strategies include:
1. Chemical water control:Use dispersants and scale inhibitors that prevent crystals from forming or adhering to the surface.
2. Cycles of Concentration:Intelligent management of the blowdown system to prevent excessive concentration of salts.
3. Inlet water treatment:Use water softeners or reverse osmosis (RO) systems to reduce the initial salts in the feed water.
4. pH control:Maintain the pH within a range that prevents the formation of calcium carbonate.
Corrosion: The invisible erosion of the structure
How does corrosion occur in a cooling tower?
Corrosion is the electrochemical degradation of metals in contact with water and oxygen. In cooling towers, various metals such as carbon steel, copper, and brass alloys are exposed to water, dissolved oxygen, carbon dioxide, and sometimes chlorides, all of which are corrosive agents.
What types of corrosion are seen in cooling towers?
- General Corrosion:Uniform, predictable erosion of the metal surface.
- Pitting Corrosion:One of the most dangerous types of corrosion that acts locally and deeply and can quickly lead to perforation of the tube wall.
- Under-Deposit Corrosion:As mentioned, deposits or any contamination on the metal surface create a galvanic cell and concentrate corrosion underneath.
- Microbiologically Influenced Corrosion (MIC):The activity of specific bacteria (such as sulfate-reducing bacteria or SRB) that directly or indirectly accelerate the corrosion process.
You can also read the article on cooling tower water corrosion based on API 571.
What are the main factors that accelerate corrosion?
- Low pH:Acidic water (pH less than 7) is highly corrosive.
- High dissolved oxygen:Oxygen is one of the main oxidizing agents in the corrosion process.
- High chloride and sulfate concentrations:These ions can destroy protective layers on the metal.
- High temperature:Increasing temperature increases the speed of corrosion reactions.
- Improper flow rate:Too high a flow can cause erosion-corrosion.
What are the corrosion control strategies?
Use of corrosion inhibitors:Chemicals that prevent water and oxygen from directly contacting the metal by forming a protective layer on the metal surface.
pH control:Maintain the pH in the neutral to slightly alkaline range (usually 7.5 to 9.0) to create a stable oxide layer on carbon steel.
Oxygen removal:In some systems, the use of oxygen scavengers can be effective.
Correct choice of materials: use of more resistant metals in sensitive parts.
Biofouling: The Underwater City of Microbes
What exactly is biofouling?
Biofouling is the growth and accumulation of microorganisms such as bacteria, algae, and fungi on wet surfaces within a cooling tower system. These microbes secrete adhesive substances, creating a sticky layer called biofilm, which acts as a base for further growth.
Why is biofouling a serious problem?
Reduced heat transfer:Biofilm acts as a thermal insulator and reduces system efficiency.
Reduced water flow:Microbial growth can block water flow paths.
Accelerated corrosion (MIC):Biofilm creates anaerobic conditions beneath it, which are ideal for corrosion-causing bacteria such as SRB.
Health hazards:The growth of pathogenic bacteria such as Legionella in cooling towers is one of the most serious health hazards, causing Legionnaires' disease.
How to control biofouling?
Controlling biofouling requires a comprehensive microbiological management program:
1. Oxidizing Biocides:Such as chlorine, sodium hypochlorite (whitewash) or chlorine dioxide, which directly kill microbes.
2. Non-Oxidizing Biocides:Such as DBNPA or isothiazolinones, which have different mechanisms of killing microbes and are usually used in rotation to prevent microbial resistance.
3. Physical cleaning:Regular flushing of the system to remove biofilms that have formed.
4. Nutrient control:Reducing nutrients such as phosphorus and organic matter in the feed water.
5. Filtration:Use appropriate filters to remove suspended particles that act as a substrate for microbes to adhere to.
Frequently Asked Questions (FAQ)
Are these three problems related?
Yes, absolutely. These three enemies are in constant interaction with each other. For example, sediment can provide a substrate for microbial growth (biofouling) and both can lead to localized corrosion. Therefore, a comprehensive water treatment program must target all three simultaneously.
Can these problems be prevented without chemicals?
Physical methods such as advanced filtration, UV radiation or electromagnetic systems exist, but in most high-capacity industrial systems, the use of chemicals is still an effective, reliable and more economical solution. A combination of physical and chemical methods often gives the best results.
How do we know which problem is dominant in our system?
Accurate identification of the type of problem requires laboratory analysis of the system water, examination of sediment or corrosion samples and sometimes microbiological tests. Guesswork can lead to choosing the wrong solution and wasting money.
Conclusion: Cooling Tower Health, Whole System Health
Sedimentation, corrosion, and biofouling are three enemies that, if ignored, can drastically increase operating costs and drastically reduce the useful life of equipment. Combating these three challenges requires a scientific, comprehensive, and continuous approach based on a deep understanding of water chemistry and system physical processes.
Rather than reacting to problems after they occur, it is better to implement a data-driven preventive and maintenance program. This not only prevents costly breakdowns, but also keeps the system’s energy efficiency optimal and ensures a healthy work environment.
If you need expert advice to identify the type of sediment or the optimal selection of chemicals, Abrizan’s experts, with over 20 years of experience in advanced laboratories, are ready to provide customized solutions to various industries.
share :













Submit your opinion
Your email address will not be published.