The inhibition rate of industrial mitreh solution against corrosion of carbon steel and stainless steel in DM environment

Abstract
When the tendency of the metal to corrode and the tendency of the environment to corrode are unavoidable, the corrosion rate of the metal or the corrosion of the environment can be determined relatively. With this information, it will be possible to estimate the corrosion rate of the equipment and the time required for repairs. The purpose of this study is to determine the effect of industrial mitreh solution in reducing the corrosion rate of widely used metals that are exposed to industrial water environments. The results showed that using mitreh solution, the corrosion of metals made of plain carbon steel and stainless steel in DM water is reduced to a high extent.
Introduction
Metal corrosion is one of the effective factors in the destruction of metal structures used in industry, which annually imposes huge repair costs if corrosion is not controlled in various industries. Among the metals widely used in industries are plain carbon steels and stainless steels, which are mainly exposed to corrosive industrial environments. Water is one of the important fluids used in various industries, which usually causes severe corrosion of metal equipment if its chemical composition is not controlled. One of the methods of reducing the rate of metal corrosion is the use of corrosion inhibitor chemicals in the fluid environment used in industries. Industrial mitreh solution is one of these corrosion inhibitors, the performance of which in reducing the corrosion rate of plain carbon steels (1010 and 1080) and stainless steel in industrial water environments has been investigated in this study. One of the cheap and common methods of examining the corrosion rate of metals is couponing under conditions almost similar to industrial operating conditions. The corrosion behavior of these metals has been investigated through couponing and measuring coupon weight loss in a simulated environment with cooling towers used in industries in the presence and absence of an industrial mitreh scale and corrosion inhibitor solution.
Procedure
The general scheme of the pilot used in the investigation of metal corrosion is given in Figure (1). As can be seen, the pilot consists of a cooling tower with a capacity of 80 lit and a water circulation circuit (corrosion rack circuit). The outlet of the circuit is connected to the inlet of the cooling tower and its inlet to the outlet of the cooling tower.

Figure (1): General diagram of the pilot used to study metal corrosion.
The details of the corrosion circuit are given in Figure (2). To prevent disturbing reactions, all parts of the corrosion circuit have been selected to be made of PVC. As can be seen, the corrosion circuit has three coupon sheaths (the place where the coupon is installed with bolts and nuts). A glass section is installed in the coupon sheath to observe the condition of the coupon during the test and has a protective cover to prevent direct light contact and the growth of biological agents. The coupons used are made of 1080 steel, 1010 steel and stainless steel, which are placed in sheaths numbered 1 to 3, respectively. The water used in this pilot was DM water and its temperature was controlled at 34 degrees Celsius by thermal elements while passing through the corrosion circuit.
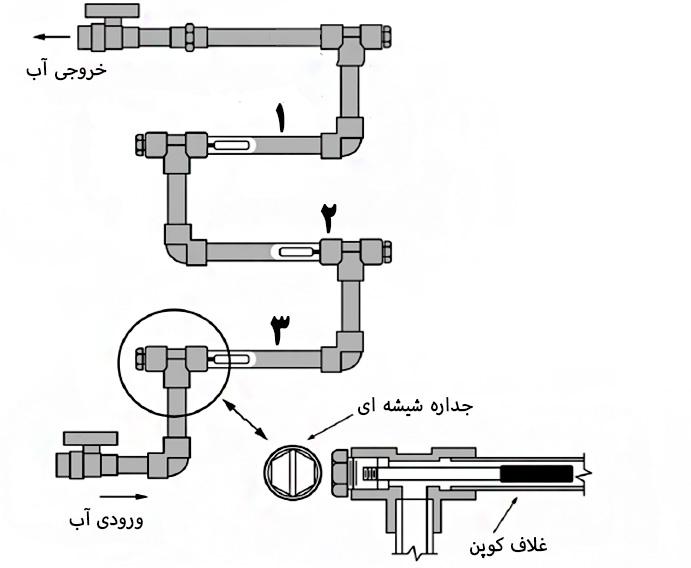
Figure (2): Details of the water circulation circuit (corrosion circuit)
The coupons were exposed to water flow (without the presence of inhibitor) for four weeks and then in the presence of inhibitor at a concentration of 35 ppm at a rate of 40 lit/min for the next four weeks, and their corrosion rate was measured in mpy by measuring the coupon weight loss at the end of each week. ASTM D2688 was used to examine the coupon corrosion rate and ASTM G1-90 was used to measure the weight loss of the samples. The complete test method was presented in detail in a report at the beginning of the pilot, which is also attached to this report.
Results and Discussion
The changes in pH and phosphate ion concentration in the medium in the presence and absence of the inhibitor are shown in Figure (3). As can be seen, in the absence of the inhibitor, the pH of the medium decreased slightly with time, which can be attributed to the interaction (interaction) between the water in the cooling tower circuit and the surrounding corrosive atmosphere. In addition, the presence of the inhibitor in the medium caused the pH to increase slightly and to be in the neutral range, and also the phosphate ion concentration in the medium increased. This phenomenon can be attributed to the phosphate base of the chemical compound of the inhibitor used. The slight changes in pH and phosphate ion concentration in the medium with time indicate the chemical stability of the inhibitor system in the aqueous environment.
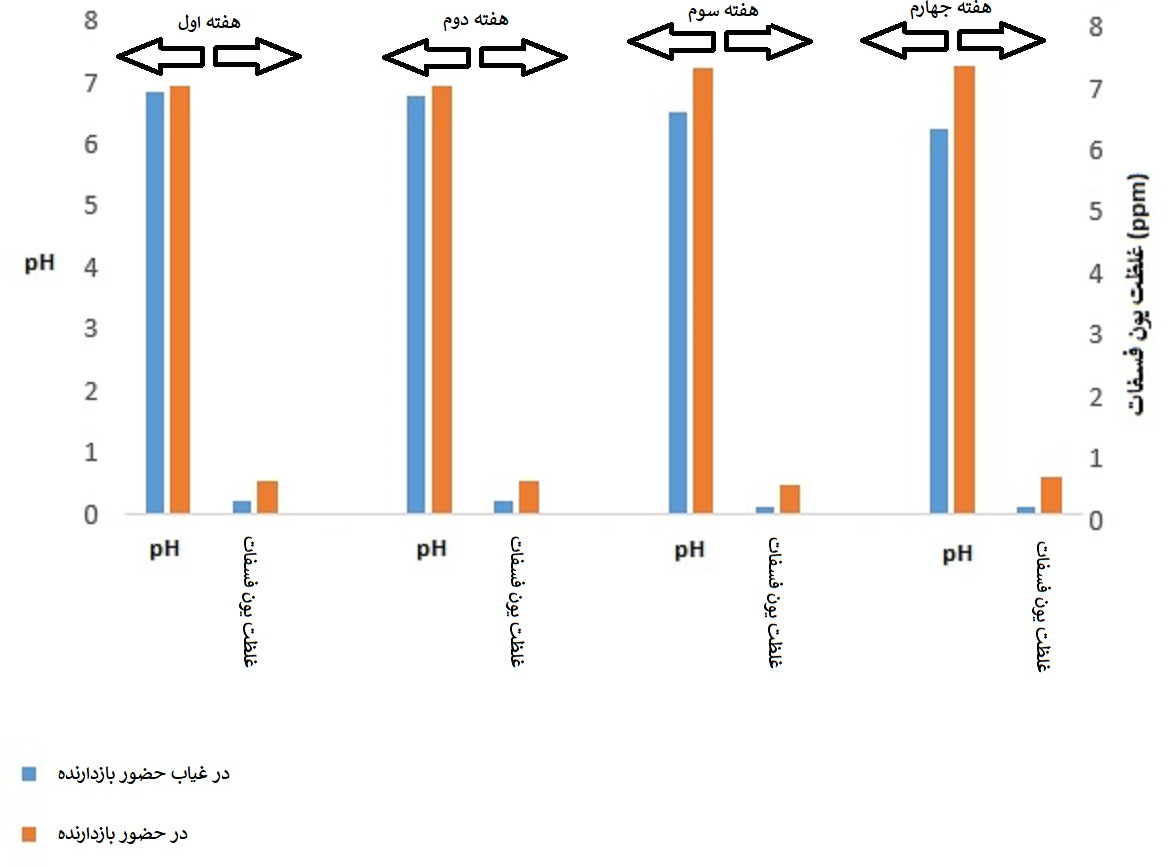
Figure (3): Changes in pH and phosphate ion concentration in the medium over time
(The reported numbers are the average of measurements taken on the corresponding days of each week)
Carbon steels are those steels in which carbon is the main alloying element and elements such as manganese, silicon and aluminum are present in small amounts and are added only for quality purposes (deoxygenation, inoculation, etc.). Carbon plays a fundamental role in increasing the strength of steels, and this strength largely depends on the amount of carbon in the alloy. Carbon steels are widely used in industry and are usually used in annealed or normalized state. Low-carbon steels are also widely used in the electrical industry due to their magnetic conductivity properties. In such alloys, general corrosion is more common and the metal surface corrodes uniformly. The damage caused by this corrosion will continue until the final destruction of the metal part. According to tests conducted at the Petroleum Industry Research Institute and also considering the effect of mitreh in this test, it is quite evident that the industrial mitreh anti-fouling and anti-corrosion solution greatly reduces the corrosion rate in plain carbon steels and can be introduced as the best inhibitor for reducing the corrosion rate of equipment made of this alloy against water.
Stainless steels are chromium alloy steels that are widely used due to their good corrosion resistance in aqueous environments. Chromium narrows the austenite range while expanding the ferrite range. When the chromium content is greater than 11%, this element increases the passivity of iron alloys and improves corrosion and oxidation resistance, which distinguishes these alloy groups from other alloys, so steels with a chromium content of 10 to 12% are introduced as stainless steels. In most cases, the naked eye is sufficient to distinguish the type of corrosion. Among the types of corrosion, 5 unique types can be stated for stainless steels, but they all have more or less similarities. These are: crevice corrosion, pitting, intergranular corrosion, stress corrosion, and abrasion corrosion. Industrial mitreh solution with special performance prevents all types of corrosion that lead to holes and pitting on the metal surface. As shown in the results, the corrosion of the stainless steel coupon was reported to be 0 mpy.
The results of the coupon corrosion rate test in DM water environment in the presence and absence of inhibitor are shown in Figure (4). As can be seen, in the absence of inhibitor, the corrosion rate of the samples was very low in the first week and increased in the following weeks. This could be due to the increased corrosiveness of the DM water environment due to contact with the corrosive atmosphere of the surrounding cooling tower over time. Also, the corrosion rate of stainless steel was less than 1 mpy, indicating a negligible corrosion rate of stainless steel in DM water.
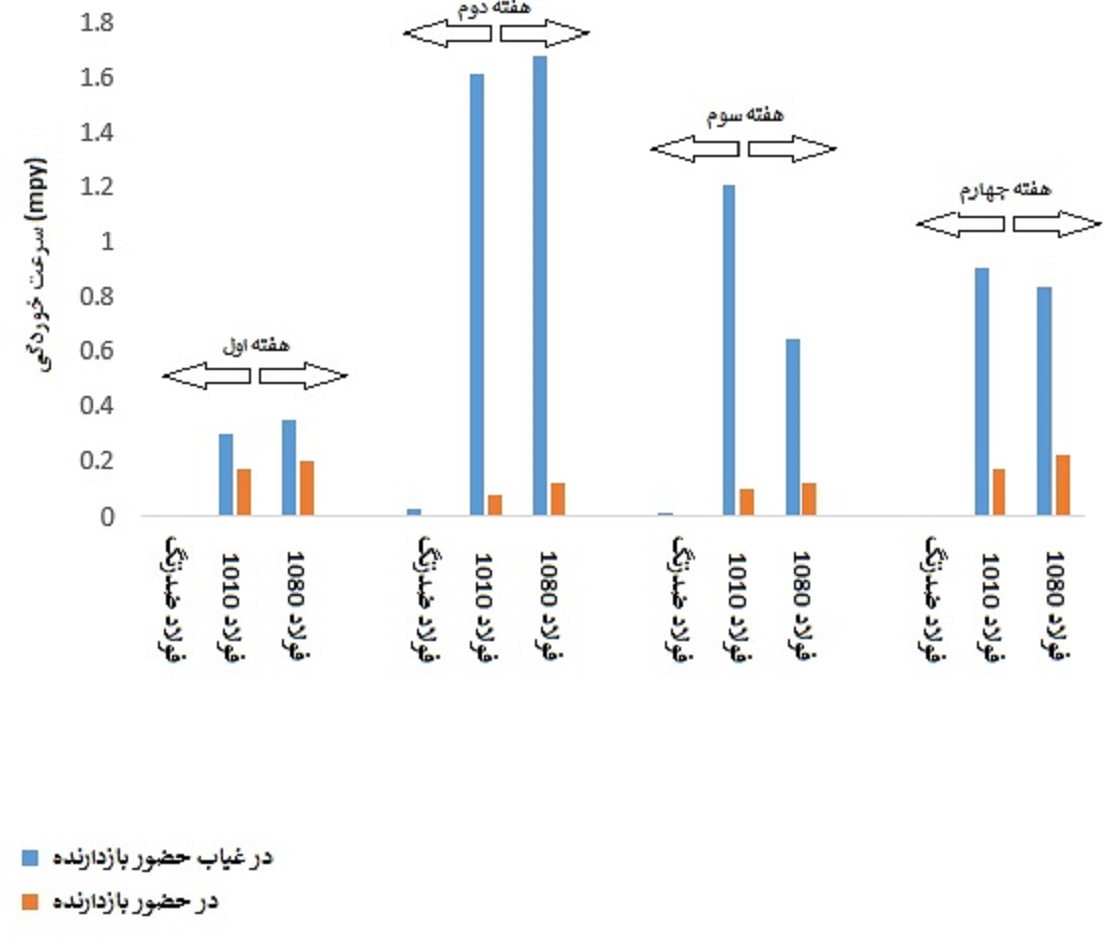
Figure (4): Corrosion rate of coupons in DM water environment in the presence and absence of inhibitor
By comparing the corrosion rate of the samples in the presence and absence of the inhibitor in the environment, it is clear that the use of the industrial Mitreh corrosion inhibitor solution has reduced the corrosion rate of 1010 and 1080 plain carbon steels to values below 1mpy and has reduced the corrosion rate of stainless steel to 0mpy. Also, the corrosion rate values in the second and third weeks are lower than the first week, which indicates that the inhibitor solution needs time to stabilize in the environment and reduce the corrosion rate.
Conclusion
The corrosion rate of plain carbon steels 1010 and 1080 and stainless steel in DM water environment with and without the presence of an inhibitor was investigated. The results showed that the corrosion rate of plain carbon steels in DM water environment is reduced to values below 0.1mpy when using industrial mitreh anti-scaling and anti-corrosion solution as an inhibitor, which indicates that the corrosion of this type of metal is stopped in the presence of this type of inhibitor in DM water environment and the unique performance of this solution. Also, the corrosion rate of stainless steel in DM water environment is negligible (values close to 0.01mpy), but the behavior of this metal based on scientific studies shows that this corrosion without a corrosion inhibitor increases logarithmically over time, but nevertheless, the use of industrial mitreh solution as a corrosion inhibitor in the environment has reduced the corrosion rate of stainless steel to 0mpy and completely protected the metal. This field pilot and review of multiple daily and weekly tests show the highly desirable performance of industrial mitreh solution in reducing corrosion rates to a high degree.
share :













Submit your opinion
Your email address will not be published.