Water vapor and its properties

What is steam? A comprehensive guide to steam types, thermodynamic properties, and industrial applications
Steam is not just a physical phenomenon; it is also one of the most important energy sources in various industries. From power plants to food production lines, steam plays a key role in heat transfer and power generation. In this article, we will thoroughly review the types of steam, related thermodynamic terms, saturated steam table, and its practical applications.
How is water vapor formed?
When we heat water, heat energy increases the kinetic energy of the molecules. At a certain temperature, called the boiling point or saturation temperature, the water molecules gain enough energy to break away from the surface of the liquid and turn into a gaseous phase (steam).
At atmospheric pressure (1 bar), this temperature is 100°C. At this point, bubbles of steam form within the liquid and rise to the surface. However, the temperature of the steam and water during boiling is the same, but the internal energy (enthalpy) of the steam is much greater than that of liquid water.
Note:Water vapor has different boiling points at different pressures. The higher the pressure, the higher the boiling point.
What is Saturated Steam?
Saturated steam is steam that is in thermodynamic equilibrium with its liquid water. That is, at a given pressure, the temperature of the steam is exactly the same as the boiling point of water.
Characteristics of saturated steam:
- Contains the maximum transferable energy for heating.
- In contact with colder surfaces, it immediately condenses and releases its latent heat.
- In industry, it is most commonly used in heating systems.
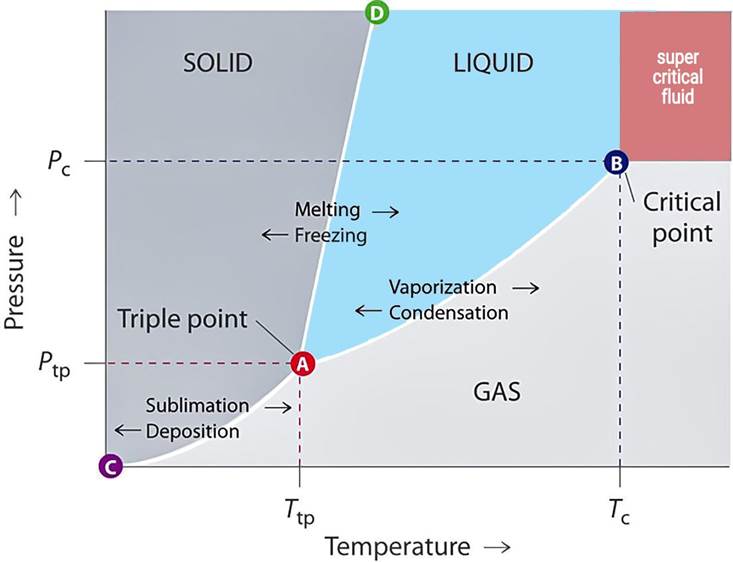
Enthalpy and Latent Heat of Vaporization
In the process of converting water to steam, the temperature of the substance does not change, but a lot of energy is absorbed. This energy is called the latent heat of vaporization or enthalpy (hrg) of vaporization.
At atmospheric pressure:
- Enthalpy of water (hf) ≈ 419 kJ/kg
- Enthalpy of vaporization (hfg) ≈ 2257 kJ/kg
- Total enthalpy of saturated steam (hg) = hf + hfg ≈ 2676 kJ/kg
This heat is returned to the environment when the steam condenses, and this property makes saturated steam the best heat conductor.
Steam Tables
A saturated steam table is a collection of experimental data that shows the thermodynamic properties of steam (such as temperature, pressure, enthalpy, specific volume) at different pressures.
Why is steam produced at high pressure?
- At 7 bar pressure, saturation temperature ≈ 170°C
- Enthalpy of water (hf) ≈ 721 kJ/kg
- Enthalpy of vaporization (hfg) ≈ 2048 kJ/kg (less than atmospheric pressure)
As pressure increases:
- Specific volume of steam decreases → Pipes and valves become smaller and more economical.
- Energy transfer capacity increases at constant volume.
T, °C | P, kPa | P, mbar |
10 | 1.2 | 12 |
35 | 5.6 | 56 |
45 | 9.5 | 95 |
Steam properties at pressures of approximately 1, 5 and 10 bar
What is Flash Steam?
Flash steam is a type of saturated steam that is produced without adding heat, only by reducing the pressure of hot water (condensate).
Practical example:
- 1 kg of condensate at 5 barg (temperature ≈ 159°C) has an enthalpy of ≈ 671 kJ/kg.
- If this condensate is discharged to atmospheric pressure (0 barg), the enthalpy of water at this pressure is only 419 kJ/kg.
- The energy difference (671 – 419 = 252 kJ) causes part of the water to evaporate → Flash steam is produced.
This phenomenon is seen in flash tanks and the outlet of steam traps and creates significant energy savings.
What is Superheated Steam?
If saturated steam is heated above its saturation temperature, superheated steam is obtained. This steam:
- is dry (free of water droplets)
- its temperature is higher than its boiling point at the same pressure
- has a higher energy storage capacity, but a lower heat transfer coefficient than saturated steam
Degree of Superheat
The difference between the temperature of superheated steam and the saturation temperature at the same pressure is called the degree of superheat.
Example: At 10 bar, saturation temperature = 180°C. If the steam is heated to 200°C, the degree of superheat = 20°C.
Applications of Superheated Steam
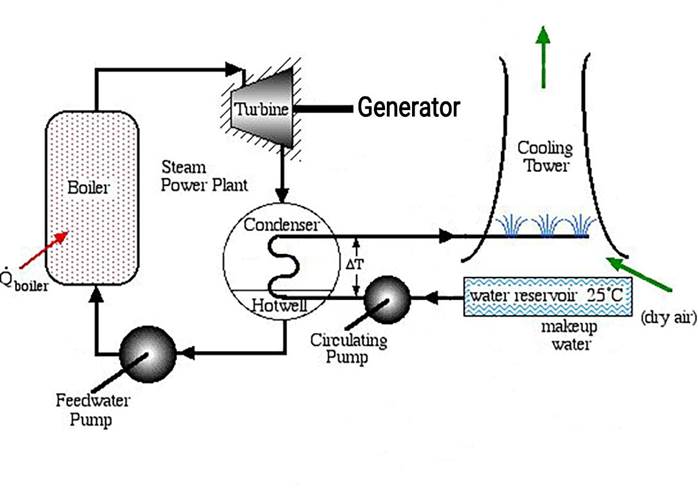
1. Steam Turbines
- Superheated steam is used at the turbine inlet to prevent the formation of water droplets during expansion.
- Water droplets can corrode turbine blades and cause destructive impacts (water hammer).
2. Increased thermal efficiency
Using thermodynamic cycles such as the improved Rankine cycle, superheated steam increases the efficiency of power plants.
3. Drying and chemical processes
In some processes where the absence of moisture is required, superheated steam is an ideal option.
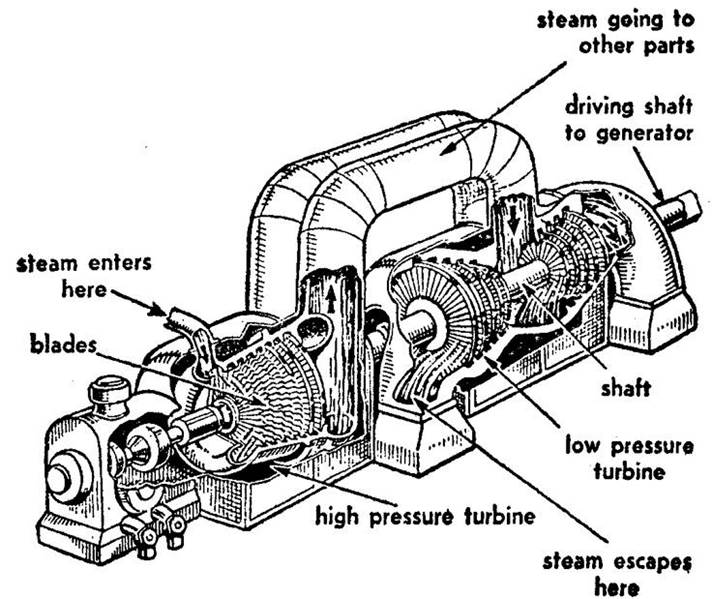
Steam Turbine Schematic
Subcooled Water
Water that is at a temperature below the saturation temperature (at a given pressure) is called subcooled water. This water has not yet reached its boiling point and needs to receive sensible heat to convert to steam.
Why is saturated steam better for heating than superheated steam?
- Saturated steam releases a lot of latent heat during condensation → Very efficient heat transfer.
- Superheated steam behaves like a hot gas and has a low heat transfer coefficient → Not suitable for heating.
Therefore, saturated steam is preferred in industrial heating systems.
Conclusion: Which type of steam is suitable for which application?
Steam type | Main application | Key feature |
Saturated steam | Heating, sterilization, cooking | Highly efficient heat transfer |
Superheated steam | Steam turbines, drying | Dry, moisture-free, high energy |
Flash steam | Energy recovery from condensate | Free steam generation without fuel |
❓❓❓ Frequently Asked Questions ❓❓❓
❓ Is the boiling point of water higher or water vapor?
Many people are faced with this question: "Is the boiling point of water higher or water vapor?" The scientific answer to this question is seemingly simple, but its precise understanding requires familiarity with the concepts of thermodynamics and the states of matter.
At standard atmospheric pressure (1 atmosphere or 101.3 kPa), the temperature of boiling water and water vapor is both 100 degrees Celsius (°C). This means that at the moment when water reaches its boiling point, both the boiling liquid water and the resulting vapor are at the same temperature.
But the main difference is in latent heat, not temperature. To convert liquid water to vapor, additional energy (known as the latent heat of vaporization) is required, which changes the molecular structure of water from a liquid to a gaseous state without increasing the temperature.
❓ So why is water vapor more corrosive?
Although steam and boiling water have the same temperature, water vapor has the ability to transfer more energy to the skin or surface. This is because when it comes into contact with a colder surface (such as human skin), the steam condenses immediately, releasing its latent heat. This process causes more severe burns than boiling water—not because of the higher temperature, but because of the greater amount of energy it transfers.
❓What if the pressure changes?
Under non-standard pressure conditions (such as at high altitudes or in industrial boilers), the boiling point of water changes. At higher pressures (such as in industrial systems), water can boil at a temperature higher than 100°C, and the resulting steam will also have a higher temperature. But in any case, at phase equilibrium, the temperatures of the boiling water and the resulting steam are the same.
Have questions? Or need advice on steam systems?
If you need help designing, optimizing, or troubleshooting your steam systems, contact our experts. We help you reduce energy consumption and increase system efficiency by providing solutions based on accurate thermodynamic data. ☎️☎️☎️
share :












Submit your opinion
Your email address will not be published.