What is Nitrate?
Nitrate is a compound that occurs naturally when nitrogen combines with oxygen or ozone. It is a polyatomic ion with the chemical formula −NO3, also known as the nitric acid ion or nitrate ion, and is in the group of inorganic compounds known as the nonmetallic nitrates. These are inorganic nonmetallic compounds that contain nitrate as the largest group of oxygen-containing anions.
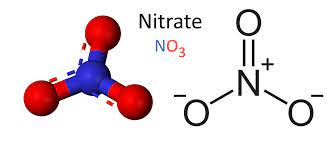
- Salts containing this anion are called nitrates.
- It is a common component of fertilizers and explosives.
- Almost all nitrates are soluble in water.
The effect of nitrates on health
High amounts of nitrates in drinking water can be dangerous for health, especially for babies and pregnant women. Nitrates are also produced in large quantities by plants and animals and are released in industrial and automotive smoke and exhaust.
Can you remove nitrate from water by boiling water?
Heating or boiling water does not destroy nitrates. Since some water evaporates during the boiling process, the nitrate concentration can increase if the water is boiled. Mechanical filters or chemical disinfection, such as chlorination, do not remove nitrates from water.
Nitrate Removal Processes from Water
Nitrate may be successfully removed from water using the following treatment processes:
- Ion exchange
- Distillation
- Reverse osmosis
Cracking due to stress corrosion cracking (SCC) in nitrate solutions
Nitrates were first identified as agents of intergranular cracking in NH4NO3-containing evaporation equipment. Stress corrosion cracking has also been observed in low and medium carbon steels in the fertilizer industry and in equipment used in the production of sodium nitrate. Calcium nitrate, ammonium nitrate, and mixtures thereof are more corrosive. Low carbon steel cracks in 60% sodium nitrate solutions, but the failure time is much longer.
The susceptibility of steel to stress corrosion cracking in nitrate solutions is affected by concentration and temperature. Increasing nitrate concentration reduces the SCC failure time and the threshold stress level. SCC can occur at low nitrate concentrations in boiling solutions, and increasing the concentration beyond a certain level does not affect the susceptibility of SCC in such solutions. On the other hand, increasing the temperature reduces the failure time.
Abrizan Company produces nitrate-based anti-fouling and anti-corrosion solutions
The laboratories of Abrizan Industrial Research Company located in Fars Science and Technology Park are capable of measuring various water parameters, including nitrate, using advanced devices and equipment.