Efficiency of cold mitreh solution in removing scale from open circuit cooling systems

Abstract:
In many industrial devices, heat transfer is an important issue that affects the efficiency of that device. The process of heat exchange between two fluids with different temperatures that are separated by a solid wall and occurs in many engineering applications. Due to the wide use of heat exchangers, research on them has a long history. Therefore, finding ways to improve the design and performance of heat exchangers and increase the heat transfer efficiency in these exchangers is of great importance. However, these activities must be continuously increased due to the energy crisis. Among the very important and costly problems that every industry faces are the formation of deposits on the surface of the heat exchanger during operation, which increases the resistance to heat transfer between the two fluids and created in the systems. In the industry, different methods and materials are used to reduce these deposits, some of which are used to prevent the formation of deposits, some to remove deposits, and some to create thin layers to protect metals.
The purpose of the present study is to investigate the effectiveness of the MDA0102 scale-out mitreh solution in industrial water environments according to the NACE standard in two environments of calcium carbonate and calcium sulfate and compare it with acid washing. According to this study, this solution appears to be a non-corrosive solution, unlike the solutions used in washing water that greatly increase corrosion. Also, the couponing results indicated that the presence of the scale-out mitreh solution will prevent further damage to machinery by forming a thin layer on the heat-transferring surface. The results of water analysis tests show that the amount of suspended and soluble solid particles in water is much higher after increasing the scale-out, which indicates that this place has a very high power in dissolving sediments. Another important point is the use of the waste of this solution in green space irrigation.
Introduction
Water is the source of life and the continuation of life of living beings depends on it. Water is also a fundamental factor in the emergence of human communities and civilizations. Today, the total volume of water on Earth, which is the source of life for all creatures, has remained almost constant and unchanging since its formation. These water resources were formed about 4.5 billion years ago. The total amount of water on Earth is 1650 million cubic kilometers, of which approximately 97 percent is unusable for human consumption and only three percent is freshwater resources, and a total of 78 percent of freshwater resources are not directly available because they are either in glaciers and groundwater tables, or are polluted. In the past century, with the increase in population and the rise in living standards, economic growth and industrial advances and agricultural development, water consumption has increased, and this has led to a decrease in per capita water. Currently, water consumption has tripled, of which 230 percent is due to population growth and the rest is the result of increased per capita water consumption. Over the past century, the exploitation of world water resources has increased sevenfold and industrial consumption has increased 30fold, and the demand for water is increasing by 2.3 percent each year. The average rainfall in Iran is 248 millimeters or 413 billion cubic meters. This is one-third of the average rainfall in the world. Thus, while one percent of the world's population lives in Iran, Iran's share of renewable water resources is only 36 percent. Of the 413 cubic meters of annual rainfall, 269 cubic meters are lost in various forms. Given that 92 percent of the water consumed in the country is related to the agricultural sector, the average global water consumption in the agricultural sector is 70 percent, which shows that Iran consumes 22 percent more than the global average. Annually, 95 billion cubic meters of water are consumed in the country, of which 92 percent is consumed in the agricultural sector, 6 percent in the rural and urban drinking and domestic sectors, 1.5 percent in the industrial sector, and 5 percent in the public sector and parks. The consumption of 92% of water in the agricultural sector is very high because the average water consumption in the world is 70% in the agricultural sector and some countries have even succeeded in reducing this consumption to 50%. The average water consumption in the drinking sector in the world is 10 to 12%, while in the country this rate is 6%. However, water consumption in the industrial sector is very low, so the water consumption pattern in the industrial sector should be given priority. However, this has caused numerous problems in the formation of sediment, which should be examined and measures should be taken to control and prevent it, to the extent that this change in attitude leads to a change in behavioral patterns and prevention of sediment formation and hardness control replaces acid washing. The direct relationship between water resources and sediment directly affects each other, meaning that the more abundant and available water is, the easier it is to control and maintain facilities with technical knowledge, and the running costs of industrialists are also reduced. Now, considering the very limited water resources and the hardness of the water due to the limestone wells that constitute most of the water resources of industrialists in Iran, it is appropriate to replace old devices with modern control and maintenance methods and, while ensuring high efficiency in all heating and cooling systems, take greater care in protecting the facilities against corrosion.
Definition of the word scale:
When water is used as a thermal fluid, the salts and hardness in the water are separated due to temperature shock and settle on the surfaces, forming scale. These scales are one of the most important factors of erosion and corrosion in heating and cooling facilities. In addition to causing erosion and corrosion, the presence of scales also wastes a significant amount of energy due to lack of heat exchange and reduces the efficiency of the facility. The effect of scale on energy loss is a topic that has received more attention today, so that the annual cost of energy loss due to scale is estimated at 45 billion dollars in all countries, which is equivalent to 2% of the world's net production.
Problems with sediment formation in facilities
Increased sedimentation leads to problems and dilemmas, the most important of which are the following:
- Severe reduction in system heat transfer
- Reduction in the speed and amount of water circulating in the system
- Corrosion of boilers and other heating systems
- Lowering the quality of produced steam
- Waste of chemicals
- Leaving stains on products, food and textiles
- Increasing the amount of heat and energy required for cooling and heating operation
- Severe reduction in system efficiency to remove residual sediments
Problems caused by sediment in the cooling tower
- Reduced evaporation efficiency and reduced cooling tower capacity
- Clogging and clogging of the cooling tower packing
- Formation of sediment in the cooling tower nozzle and its clogging
- Clogging of pipes connected to the cooling tower
- Increased water and electricity consumption in the cooling tower
With any type of treatment system, facility operators will encounter a significant amount of sediment after a while, which they must inevitably dissolve. The remaining sediment will be removed by acid. Various chemicals are used in different industries, but there are many similarities in the general method of use.
Ways to remove sediments
As described in previous discussions, different methods are used to prevent the entry of hard water and sedimentation, but the complete inefficiency of these methods causes sediment and the resulting harm. Therefore, it is obvious that the sediments created must be removed from the system. Below, we compare the types of methods with each other.
- Acid washing
- Physical methods (spiking, drilling, etc.)
- Non-acidic chemicals
Disadvantages of acid washing
The main disadvantages of using acid, which is accepted as the last solution for controlling deposits, are as follows:
- Dangers and lack of proper safety for the consumer and the creation of poisoning caused by the gas produced during use
- Severe corrosion in pipes and installations due to problems caused by the lack of precise control of acid during and after washing, which sometimes continues until the destruction of the installation after several uses
- Lack of standards and sufficient and uniform information about how to select the required materials in different systems and the amount of consumption, which has led to irregularity in its use in various industries
- Production of contaminated wastewater and severe environmental pollution due to the expansion of consumption and penetration of these materials into the soil surface and groundwater
- Complete shutdown of the system during acid washing
- Failure to completely remove deposits from the installation
Disadvantages of Physical Methods
Among the disadvantages of physical methods of sediment removal such as spiking, drilling, brushing, etc., the following can be mentioned:
- Incomplete sediment removal
- Deformation of facilities
- Physical damage and injuries to systems
- Lack of performance standards
- System shutdown (shut down-overhaul)
Non-acidic chemicals
These products are considered to save energy, water, increase productivity, and reduce depreciation. This very valuable material with high strength and durability eliminates previous sediments in the system and, more importantly, makes the system safe for a long time against new sedimentation and corrosion.
These materials are non-acidic and non-corrosive and will not cause any harm to the facilities and equipment. In addition to preventing energy waste, this method is also very economical in terms of economy.
Some of the important advantages of non-acidic chemicals
- Prevention of scale formation Prevention of corrosion
- Non-acidic non-corrosive
- Non-stop scale removal Complete scale removal system of the facility
- Environmentally friendly Wide range of operation in the industry
The scale removal solution introduced to this system is a cold Mitreh scale removal agent produced by Abrizan Company called MDA0102, which has been used many times for scale removal in open circuit cooling towers. In this project, the aim was to investigate the efficiency of the performance of this scale removal agent in industrial water environments and compare it with acid washing and how to investigate these behaviors in a simulated environment with cooling towers used in industries, by comparing the results of coupon tests and water analysis in the presence and absence of the scale removal agent.
Method of work
The general scheme of the pilot is a cooling tower with a capacity of 80 liters and a water circulation circuit (recirculation circuit). The output of the circuit is connected to the inlet of the cooling tower and its inlet is connected to the outlet of the cooling tower.

Figure (1): General diagram of the pilot used in the sedimentation study using MDA0102
To investigate the behavior and efficiency of the sediment vector produced by Abrizan Company under the name MDA0102, a water sample had to be prepared first, which contained high amounts of sediment able compounds such as calcium and magnesium carbonate, calcium phosphate, calcium and magnesium sulfate, and silica. The most common of these are calcium phosphate and calcium carbonate. For this purpose, a heavy water sample or high-hardness water sample containing a lot of solid particles was taken from the Karun River water, and after cleaning and purifying the cooling system, it was introduced into it. The purpose of this work is only to create sediment in preparation for sediment removal. There is no specific standard for creating sediment, and this work was done only for sedimentation to provide a specific volume of sediment for sediment removal.


Figure (2): Preparation of heavy water and cleaning of the pilot before starting the experiment
Coupons made of C1010 iron and copper to measure the corrosion rate and a C1010 deposition coupon to measure the deposition rate were first weighed and then installed in the corrosion rack (Figure (3)).

Figure (3): Weight measurement of three iron and copper-based corrosion and fouling coupons for testing
The coupons were exposed to water flow for a period of one month and their corrosion rate in terms of mpy was obtained by measuring the coupon weight loss.
A sediment coupon was also used to investigate the fouling rate.
Fresh water entering the cooling tower basin was analyzed and XRD analysis was also performed, and its physical properties were measured. After the fouling was removed, analysis was also performed. Photographs were also taken of the surfaces on which fouling had formed. The desired fouling vector was used under equal fouling and settling conditions (heavy water, temperature, corrosion coupons and fouling, etc.) and its efficiency and effectiveness were compared with acid washing.
Discussion and Results
The deposition and settling of solid particles in the water circulating in the cooling tower can cause corrosion and microbial growth, as well as other such issues, and can cause numerous technical problems in the system and even change the production path in industries. These solids can be dissolved solids and suspended solids (parts that are retained by the filter).
As previously mentioned, to examine the behavior and efficiency of the MDA0102 scale remover solution, a water sample must first be prepared that contains high amounts of sedimentable compounds such as calcium and magnesium carbonate, calcium phosphate, calcium sulfate, and magnesium silica. What is targeted for sediment removal is actually the calcium and magnesium carbonate and sulfate sediments mentioned above. For this purpose, a heavy water sample or high-hardness water sample containing many solid particles was introduced into the cooling system after cleaning and purifying it. This water was obtained from the Karun River. Then, this water remains in the cooling tower system for a while until the required amount of sediment is formed in it. As can be seen in the figure below (Figure (4)), the required sediment was evident after three weeks. Initially, before using the Mitreh MDA0102 sediment vector, the sediments in the pilot cooling tower were chemically analyzed. XRD phase analysis was used to determine the type of sediments. The circulating water in the system was chemically analyzed before and after adding the sediment vector. Also, the corrosion rate in the corrosion coupons made of iron and copper, as well as the volume of sediment formed in the sediment coupon holes, and photographs of different surfaces of the cooling tower, were also taken to determine the amount of sediment removal in the cooling tower using the desired sediment vector.



Figure (4): Sediment formed in the pilot after using river water in the cooling tower
In principle, the type of deposits is considered a very important factor in terms of the intensity of adhesion to the metal surface, the heat transfer coefficient, and solubility by anti-fouling solutions. Figure (5) shows the results of the phase analysis of the deposit in question.
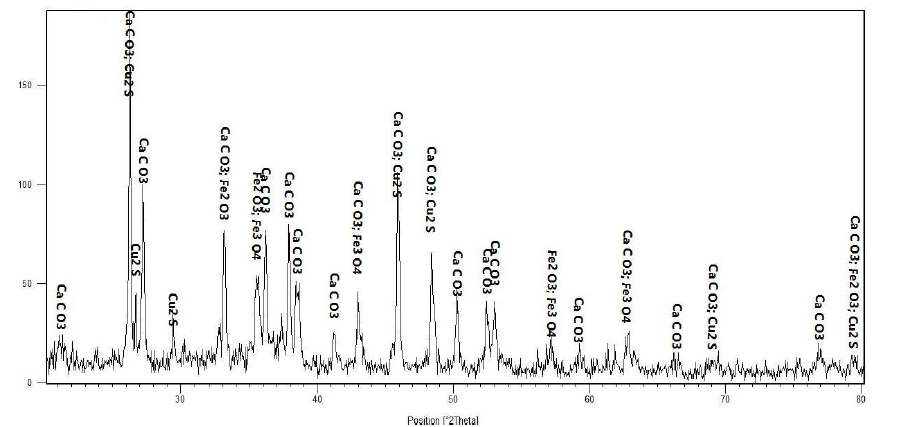
Figure (5): Results of XRD phase analysis of the deposit formed in the cooling tower
As is clear from the figure above, calcium carbonate comprises the major part of the above product. The presence of iron oxides along with copper sulfide indicates the presence of a significant amount of oxygen and sulfur, both of which are corrosive agents. The peeling of oxide layers on the surface of copper and iron metals, both of which are in direct contact with water, is not far from being expected. The speed of water movement is simultaneous with the formation of brittle and swollen oxide layers on the surface of the metals, which ultimately leads to peeling and the formation of these products in other areas and walls of the system. Especially in areas where the temperature is higher, the formation of these deposits will be much easier. As is clear from the figure above, no trace of silicate deposits is seen. In fact, this result was not far from being expected because these deposits have a high adhesion to the surface and their peeling and dissolution are not easy.
Scale Removal by Acid Washing
Acid washing is one of the oldest improvement methods in the industry for scale removal. The acid used is hydrochloric acid, which has been used in a 10% chemical washing of the pilot. This combination is one of the common compounds that is widely used in industries for scale removal. As can be seen in the tests and photographs taken of different parts of the tower and the rate of weight loss of the coupons and the corrosion rate in them, it has caused a large amount of corrosion of the coupon because the acid is highly corrosive and is able to penetrate the inactive layers where metal oxides are present and establish local anodic cells.
Conclusion:
According to the results and discussions mentioned in this study, it can be stated that the MDA0102 scale removal solution, in addition to scale removal during system operation, is able to create a protective layer to prevent scale deposition on the cooling tower and prevent corrosion, and it can be recognized as a non-corrosive scale removal solution. According to the cases seen during the test, this natural solution has no adverse effects on the human body and has eliminated the problems related to workplace safety. In addition to observing the issues related to environmental protection, due to the presence of mineral salts that can be absorbed by plants and hydroxide compounds, unlike carbonate and silicate compounds in the waste of this solution, it is possible to reduce its chlorine content by adding dechlorinating agents and use it for green spaces. However, it is correct to use common scale removers in the industry (10% sulfuric acid) for scale removal, which can be done in this method, but the cleaning intensity in this method is lower than cold mitering, and in addition, the corrosion intensity in the system is very high with this method.

Figure (6): Absence of scale in various parts of the pilot cooling tower as a result of using Mitreh MDA0102 scale remover and acid washing

Figure (7): Image of the deposition coupon after acid washing.
Table (1): Physical properties of water in the cooling tower before pickling
row | parameter | amount | unit |
1 | Cl- | 55.97 | mg/l |
2 | HCO3- | 272.56 | mg/l |
3 | Na2+ | 177 | mg/l |
4 | pH | 7.93 |
|
5 | Total Hardness | 76.56 | mg/l |
6 | Total Alkali | 238.82 | mg/l |
7 | Total solid | 510 | mg/l |
Table (2): Chemical analysis of water after acid washing
row | parameter | amount | unit |
1 | Cl- | 856.34 | mg/l |
2 | HCO3- | 405.33 | mg/l |
3 | Na2+ | 567.78 | mg/l |
4 | pH | 5.45 |
|
5 | Total Hardness | 456.67 | mg/l |
6 | Total Alkali | 567.47 | mg/l |
7 | Total solid | 3450 | mg/l |


Figure (8): Schematic of coupon corrosion after using MDA0102 sediment vector
With the collaboration of Dr. Reza Bahadori Biragani
share :















Submit your opinion
Your email address will not be published.