Membrane processes

(Membrane Processes)
Membrane processes refer to physical methods for separating a solvent from its dissolved salts using semipermeable membranes. These processes have made great progress in recent years. The history of using membranes for filtration dates back to the early 20th century. In the third decade of the 20th century, membranes were used to separate, purify, or concentrate solutions, especially fluids containing microorganisms. The evolution of this phenomenon continued over time with research on the construction of various membranes and understanding of the process, such that this process is currently considered one of the main methods of desalination of seawater.
Types of membrane processes:
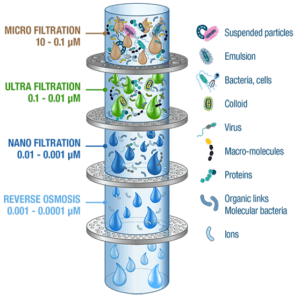
Membrane processes are named according to the size of the smallest particle that passes through the membrane under the influence of pressure force:
- (Micro Filtration)
- (Ultra Filtration)
- (Nano Filtration)
- (Reverse Osmosis – Hyper Filtration)
In a membrane process, two phases are typically separated by a third phase, the membrane. The membrane is the determinant of the mass transfer phenomenon. Each of the two phases is a solution of different components, some of which are transported through the membrane more than others. As the mass transfer process continues, one phase becomes saturated with the passing components and the other becomes depleted of these components.
The two main characteristics of transport exerted by membranes are:
- (permeability)
- (Selectivity)
All materials that act as membranes have the property of selectively passing different substances.
Membrane processes can utilize a variety of driving forces for separation. Mass transfer across a membrane can be accomplished by diffusion or convection phenomena. Convection mass transfer is driven by differences in electrical potential, concentration, pressure, or temperature.
Membrane processes have the following various advantages:
- Energy saving due to no phase change
- Reduced space requirements due to the compact size of the membrane modules.
- The process is faster due to the thinness of the membrane and the high mass transfer rate in it.
- Ability to operate at low temperatures, which is a great advantage for heat-sensitive solutions.
- Low investment cost compared to other methods globally.
- Ease of expanding the system.
- Ability to be manufactured in various shapes and sizes.
Membranes have a long life. Ultrafiltration membranes have been reported to have a useful life of more than two years and reverse osmosis membranes to have a useful life of more than five years. Membranes are made of various organic or inorganic materials. These materials include polymers, ceramics, metals, and liquids, with polymeric materials being the most widely used. Membrane performance is the origin of the choice of membrane material in the design of a membrane process. The performance of a membrane is determined by its physical and chemical properties.
Chemical properties and physical properties of the membrane
The chemical properties of a membrane include its reactivity with other materials, surface charge, and ability to adsorb other substances. The physical properties of a membrane include the size, shape, and type of pores in the membrane, mechanical strength, and thermal resistance. The hydrophobicity or hydrophilicity of a membrane is determined by its physical and chemical properties. The most fundamental variables that determine the physical and chemical properties of a membrane include the raw materials used, the method of construction, and the membrane modification operations.
Polymer membranes are used more than other membranes. The application of each membrane depends on the environmental conditions. For example, membranes made of cellulose nitrate or cellulose acetate are sensitive to heat, chemicals and biological materials. To prevent their deterioration, the pH of the environment must be acidic and between 4 and 6.5 and the membrane temperature must be within the normal range. Membranes produced from cellulose derivatives hydrolyze rapidly in alkaline environments. Teflon (PTFE) membranes have greater resistance compared to cellulose membranes. They are resistant to acidic and basic environments and chemicals except cyclic hydrocarbons at high temperatures. These membranes can withstand temperatures from 100 to 300 degrees Celsius. Teflon membranes are mechanically reinforced with polyethylene holders so that they do not get damaged in case of movement. Membranes made of polyvinyl chloride (PVC) in pure form or mixed with other polymers have good resistance to relatively strong acids and bases, but they soften at temperatures above 65°C.
The membrane needs to be cleaned after use for some time. This is done with chemicals. The choice of cleaning chemical depends on the type of polymer used in the membrane. For example, membranes made of polyamide, which have good resistance to solvents and are capable of being sterilized by autoclaving, are highly sensitive to chlorine in chemicals, and as a result, chlorine-containing substances cannot be used to clean these membranes.
The structure of the membrane is called membrane morphology and plays a fundamental role in how it functions.
Solid membranes have two main structures:
- (Porous membranes)
- (Non-porous membranes)
In porous membranes, the size of the pores and their distribution vary. Membranes are classified based on the size of their pores as follows:
- Large pores: Membranes with an average pore size greater than 20 nm.
- Medium pores: These are membranes whose average pore size is between 20 and 1 nanometers.
- Small pores: These include membranes whose average pore size is less than 1 nanometer.
According to the above definition, microfiltration membranes have large pores, ultrafiltration membranes have medium pores, and nanofiltration membranes have small pores. Reverse osmosis membranes are without pores.
share :













Submit your opinion
Your email address will not be published.