Measurement of ammonia NH3
آمونیاک یک ترکیب معدنی است که از اتصال یک اتم نیتروژن به سه اتم هیدروژن تشکیل شده و فرمول آن NH₃ است. این ماده به طور طبیعی از فرآیندهای باکتریایی و تجزیه مواد آلی تولید می شود. همچنین ممکن است از طریق تخلیه ضایعات فرآیند صنعتی حاوی آمونیاک و کودها به آبهای زیرزمینی و سطحی راه پیدا کند.
تعیین میزان آمونیاک
آمونیاک یک ترکیب معدنی است که از اتصال یک اتم نیتروژن به سه اتم هیدروژن تشکیل شده و فرمول آنNH₃ است. این ماده به طور طبیعی از فرآیندهای باکتریایی و تجزیه مواد آلی تولید می شود. همچنین ممکن است از طریق تخلیه ضایعات فرآیند صنعتی حاوی آمونیاک و کودها به آبهای زیرزمینی و سطحی راه پیدا کند.
آمونیاک در بسیاری از فرآیندهای صنعتی، و همچنین به عنوان کود و مبرد استفاده می شود. آمونیاک به عنوان یک گاز بیرنگ یا بصورت مایع فشرده با بوی تند مشخص می شود و قرار گرفتن در معرض آن با استنشاق، بلعیدن یا تماس اتفاق می افتد.
موارد استفاده از آمونیاک:
حدود 80 درصد آمونیاک تولید شده صنعت در کشاورزی به عنوان کود استفاده می شود. آمونیاک همچنین به عنوان گاز مبرد، برای تصفیه منابع آب و در تولید پلاستیک، مواد منفجره، منسوجات، آفت کشها، رنگها و سایر مواد شیمیایی استفاده می گردد.
تاثیر و عوارض آمونیاک بر بدن انسان:
قرار گرفتن در معرض غلظت بالای آمونیاک در هوا باعث سوزش فوری چشم ها، بینی، گلو و دستگاه تنفسی می شود و می تواند منجر به کوری، آسیب ریه یا مرگ شود. استنشاق غلظت های کمتر می تواند باعث سرفه و سوزش بینی و گلو شود.
تاثیر آمونیاک بر روی گیاهان:
همچنین آمونیوم(NH4+)یا آمونیاک بدون بار(NH3)شکلی از نیتروژن است که گیاهان آبزی می توانند آن را جذب کرده و در پروتئین ها، اسیدهای آمینه و سایر مولکول ها ترکیب کنند. غلظت بالای آمونیوم می تواند رشد جلبک ها و گیاهان آبزی را افزایش دهد. باکتریها همچنین می توانند آمونیوم بالا را به نیترات (NO3–)در فرآیند نیتریفیکاسیون تبدیل کنند که این امر باعث کاهش اکسیژن محلول می شود.
رابطه آمونیاک بامقدارpH
آمونیاک غیر یونیزه سمی است و زمانی کهpHبالا باشد غالب می شود. یونNH4+ نسبتا غیرسمی است و زمانی کهpHپایین باشد غالب می شود. به طور کلی، کمتر از 10٪ آمونیاک هنگامی کهpHکمتر از 8 باشد، در حالت سمی است. این نسبت با افزایشpHبه طور چشمگیری افزایش می یابد. تعادل بینNH3 و +NH4 نیز تحت تأثیر دما است و در هرpH، آمونیاک سمی تر در آب گرمتر نسبت به آب سردتر وجود دارد.
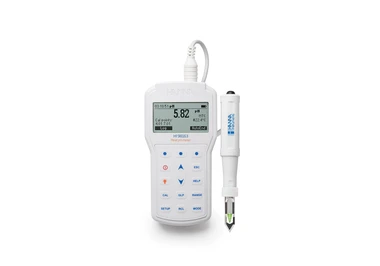
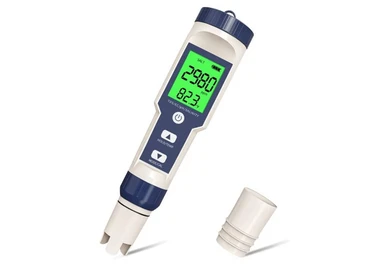
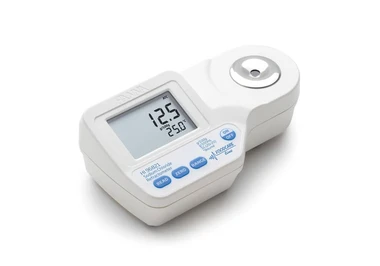
چگونه میزان آمونیاک را اندازه بگیریم؟
یک روش رنگ سنجی ساده برای تعیین آمونیاک در آب در سطحppmوجود دارد که رنگ آبی ایندوفنول در محیط بورات قلیایی (نمکی که آنیون آن مانند بوراکس حاوی بور و اکسیژن است) با استفاده از فنل و هیپوکلریت به عنوان معرف تولید می شود. مقایسه شدت رنگ با استفاده از لوله های آزمایش انجام می گیرد.
مقدار استاندارد استفاده از آمونیاک در آب آشامیدنی چقدر است؟
محدودیت های زیست محیطی آمونیاک در آبهای سطحی در ایالات متحده بین 25/. تا 5/32 میلی گرم در لیتر(ppm)است. آکادمی ملی علوم و بسیاری از کشورها استاندارد آب آشامیدنی را 5/. میلی گرم در لیتر(ppm)را پیشنهاد کرده اند.
خنثی کردن آمونیاک در آب:
حذف آمونیاک از آب دشوار است. می توان آن را با رزین تبادل کاتیونی به شکل هیدروژن، که نیاز به استفاده از اسید به عنوان احیاکننده دارد، حذف کرد. Degasification(فرآیندی برای حذف گازهای حل شده در مایعاتی که در مخازن فلزی ذخیره شده یا از خطوط لوله عبور می کنند) نیز می تواند موثر باشد.
آمونیاک یا یون آمونیوم موجود در آب غیر یونیزه هستند. به طور معمول، مقدار گزارش شده مجموع هر دو شکل است و با عنوان آمونیاک کل یا به بطور معمول تر “آمونیاک” گزارش می شود. نسبت نسبی دو شکل موجود در آب به شدت تحت تأثیرpHاست.
*آزمایشگاه های شرکت پژوهشی صنعتیآبریزان واقع در پارک علم و فناوری فارس با کادری مجرب و استفاده از دستگاه ها و تجهیزات پیشرفته قادر به اندازه گیری انواع پارامترهای آب از جمله آمونیاک می باشد.