Water softening

Sedimentation water softening processes
Softening processes are used to reduce water hardness. Using chemicals, water hardness undergoes chemical reactions and precipitates. As a result, water hardness is reduced. Water hardness is due to the presence of carbonates, sulfates, chlorides, and nitrates of the metals calcium, magnesium, iron, and aluminum.
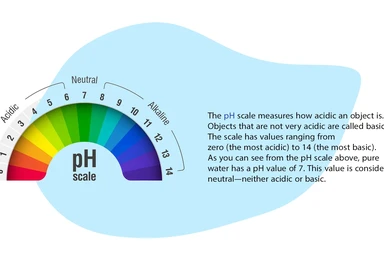
The importance of water pH measurement
pH measurement is one of the most important and common tests in water chemistry. Almost every step in water supply and wastewater treatment (e.g., acid-base neutralization, water softening, sedimentation, coagulation, disinfection, and corrosion control) depends on pH. pH is used in the measurement of alkalinity and carbon dioxide and many other acid-base equilibria. At a given temperature, the intensity of the acidic or basic activity of a solution is indicated by pH, or hydrogen ion activity.
