Water analysis

What is the Langelier Index? A Complete Guide to Predicting Water Sedimentation and Corrosion
Find out if your water is prone to scaling or corrosion with Langelier Index (LSI), Reisnar and Pocurius. A practical guide for industries and water systems

Measuring Carbonate and Bicarbonate in Water: A Complete Guide to Alkalinity Control
Learn why water alkalinity with carbonate and bicarbonate is important, how to measure it, and what solutions there are to prevent scaling.

Measuring Iron (Fe) in Water: Why Can This Common Metal Disable Industrial Equipment?
Why is iron in water dangerous? Fe measurement method, limits, impact on equipment and control solutions + accurate laboratory analysis.

Water Parameter Analysis: A Complete Guide to Water Quality Control in Industry and the Environment.
What is water parameter analysis? How to prevent scale and corrosion? Comprehensive physical, chemical and microbial guide + answers to common questions

Orthophosphate - phosphate
Orthophosphates, pyrophosphates, metaphosphates, and other polyphosphates and organophosphates are phosphorus in natural waters and wastewaters that occur as phosphates. They are found in solution, in particulate or powder form, or in the form of aquatic organisms.

The origin and importance of magnesium
Magnesium is an essential element in chlorophyll and in red blood cells. Some magnesium salts are toxic if ingested or inhaled. Concentrations above 125 mg/L can also have a laxative and diuretic effect.

The origin and importance of iron
The solubility of iron ions is controlled by the concentration of carbonate. Since groundwaters are often oxygen-deficient, soluble iron in groundwaters is usually in the form of iron salts. Upon exposure to air or the addition of oxidants, iron is oxidized to the ferrous state and can be hydrolyzed to produce the insoluble red hydrated iron oxide.

Copper and its importance
Copper exists in its elemental state, but is also found in many minerals, the most important of which include sulfide compounds (such as chalcopyrite), as well as oxides and carbonates. Copper is widely used in electrical wiring, roofing materials, various alloys, pigments, cookware, plumbing, and the chemical industry. Copper salts are used in water supply systems to control biological growth in tanks and distribution pipes and to catalyze the oxidation of manganese.

Organic matter and its measurement
There are various methods for measuring the amount of organic matter in water. These methods include measuring the volatile fraction, measuring the total solids, BOD and COD. Because measuring the volatile fraction and measuring the total solids has a relatively large error, they are more often used than measuring the biochemical oxygen demand (BOD) and chemical oxygen demand (COD) to obtain the amount of organic matter in water.

Alkalinity phenolphthalein kit
Alkalinity is a chemical measure of water's ability to neutralize acids. Alkalinity is also a measure of the buffering capacity of water, or its ability to resist changes in pH after the addition of acids. The alkalinity of natural waters is primarily due to the presence of weak acid salts, although strong bases may also contribute in extreme environments, e.g. (–OH).

Electrical conductivity of water (EC)
The electrical conductivity (EC) of water is a measure of the ability of water to conduct an electric current. This ability is directly related to the concentration of conductive ions in the water. These conductive ions are caused by the presence of inorganic substances such as chlorides, alkalis, carbonate compounds, and sulfides, and dissolved salts. Most metals are excellent conductors of electricity because of the large number of free electrons.

Water pH measurement kit
We use a pH measurement kit to quickly and easily test and measure the pH of drinking and industrial water to maintain health and prevent damage to equipment, as well as control production processes.

What is a total hardness kit?
Water hardness is caused almost entirely by calcium and magnesium ions. Other divalent and trivalent metals have a similar effect, but are not usually present in sufficient concentrations in drinking water to cause problems. High hardness prevents the formation of soap scum and can cause scale in water systems, especially boilers, cooling systems, and fresh water piping.
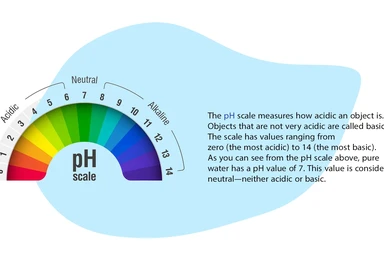
The importance of water pH measurement
pH measurement is one of the most important and common tests in water chemistry. Almost every step in water supply and wastewater treatment (e.g., acid-base neutralization, water softening, sedimentation, coagulation, disinfection, and corrosion control) depends on pH. pH is used in the measurement of alkalinity and carbon dioxide and many other acid-base equilibria. At a given temperature, the intensity of the acidic or basic activity of a solution is indicated by pH, or hydrogen ion activity.

How is chloride measured?
Chloride, in the form of the chloride ion (Cl-), is one of the most important mineral anions in water and wastewater. The salty taste varies with the chloride concentration and is dependent on the chemical composition of the water. Some waters containing 250 mg Cl-/L may have a detectable salty taste if the sodium cation is present.

Alkalinity of water and its importance
Chemically, the alkalinity of water indicates its capacity to be neutralized by an acid. Neutralization of water means that the pH of the water reaches about 4.5. The alkalinity of natural waters is due to the presence of hydroxides, carbonates, and bicarbonates.
