lithium bromide

The effect of lithium bromide on corrosion of metals
The concentration of LiBr in solution can significantly affect the rate and severity of corrosion in metals. The concentration of lithium bromide can increase the corrosion rate, affect the pH of the solution, the formation of corrosion products, the solubility of oxygen, the effects of temperature, and also cause specific metal reactions.

Refrigeration cycles
As a refrigerant circulates through a system, it goes through a number of changes of state or conditions, each of which is called a transformation. The refrigerant starts from an initial state and returns to its initial state after going through a series of transformations called a cycle. Various refrigeration cycles are used to produce refrigeration, the most important of which are the compression refrigeration cycle, the absorption refrigeration cycle, and the thermoelectric refrigeration cycle.

Refrigeration and refrigerants
Any process in which heat is absorbed is called refrigeration. In general terms, a refrigerant is a substance that acts as a cooling agent by absorbing heat from a substance.
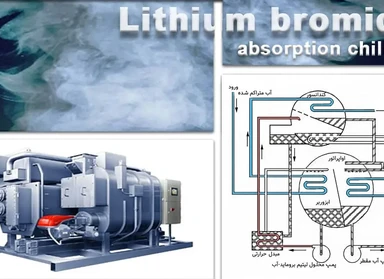
Working method of lithium bromide absorption chillers
Lithium bromide absorption chiller is one of the most common types of absorption chillers in which lithium bromide plays the role of absorbing water vapor to reduce the pressure in the evaporator chamber.
Chemical analysis of lithium bromide solution
Lithium bromide is a chemical widely used in absorption chillers. This chemical has a high moisture absorption capacity. Therefore, it is able to absorb the vapors in the system and thus return them to the liquid phase. However, lithium bromide is highly corrosive.
