Absorption chiller

Lithium Bromide Absorption Chillers: Application, Performance, and Maintenance Challenges
How do lithium bromide absorption chillers work? Why are they suitable for air conditioning systems? A comprehensive guide to their performance, benefits, limitations, and chemical maintenance.

What is an absorption chiller? A comprehensive guide to the operation, benefits and challenges of thermal cooling systems
How does an absorption chiller produce cold with heat? Differences from a compression chiller, chemical challenges and maintenance solutions + analysis of lithium bromide solution.

The effect of lithium bromide on corrosion of metals
The concentration of LiBr in solution can significantly affect the rate and severity of corrosion in metals. The concentration of lithium bromide can increase the corrosion rate, affect the pH of the solution, the formation of corrosion products, the solubility of oxygen, the effects of temperature, and also cause specific metal reactions.

What is a chiller?
Chiller is derived from the Latin word Chill, meaning to cool. Various cycles are used to create refrigeration, the most important of which are the compression refrigeration cycle, the absorption refrigeration cycle, and the thermoelectric refrigeration cycle.

Types of refrigeration systems
Refrigeration is the process of absorbing heat from one fluid and releasing it to another. The fluid can be air, water, or any other gas or liquid. In all refrigeration systems, keeping cool involves absorbing heat from a lower-temperature material and releasing it to a higher-temperature environment.
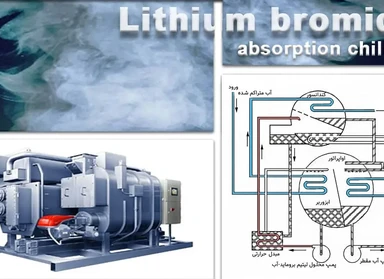
Working method of lithium bromide absorption chillers
Lithium bromide absorption chiller is one of the most common types of absorption chillers in which lithium bromide plays the role of absorbing water vapor to reduce the pressure in the evaporator chamber.
Chemical analysis of lithium bromide solution
Lithium bromide is a chemical widely used in absorption chillers. This chemical has a high moisture absorption capacity. Therefore, it is able to absorb the vapors in the system and thus return them to the liquid phase. However, lithium bromide is highly corrosive.
